Prior anti-CAFs break down the CAFs barrier and improve accumulation of docetaxel micelles in tumor
Ning Pang, Ji Li, Aning Sun, Zhenzhen Yang, Shixuan Cheng, Xian-Rong Qi*
International Journal of Nanomedicine, 2018, 13:5971-5990
Background:Abnormal expression of stromal cells and extracellular matrix in tumor stroma creates a tight barrier, leading to insufficient extravasation and penetration of therapeutic agents. Cancer-associated fibroblasts (CAFs) take on pivotal roles encouraging tumor progression.
Method:To surmount the refractoriness of stroma, we constructed a multi-targeting combined scenario of anti-CAFs agent tranilast and antitumor agent docetaxel micelles (DTX-Ms). Tranilast cut down crosstalk between tumor cells and stromal cells, ameliorated the tumor microenvironment, and enhanced the antiproliferation efficacy of DTX-Ms on cancer cells.
Results:Diverse experiments demonstrated that tranilast enhanced DTX-Ms’antitumor effect in a two-stage pattern by CAFs ablation, tumor cell migration blocking, and metastasis inhibition. Along with activated CAFs decreasing in vivo, the two-stage therapy succeeded in reducing interstitial fluid pressure, normalizing microvessels, improving micelles penetration and retention, and inhibiting tumor growth and metastasis. Interestingly, tranilast alone failed to inhibit tumor growth in vivo, and it could only be used as an adjuvant medicine together with an antitumor agent.
Conclusion:Our proposed two-stage therapy offers a promising strategy to enhance antitumor effects by breaking down CAFs barrier and increasing micellar delivery efficiency.
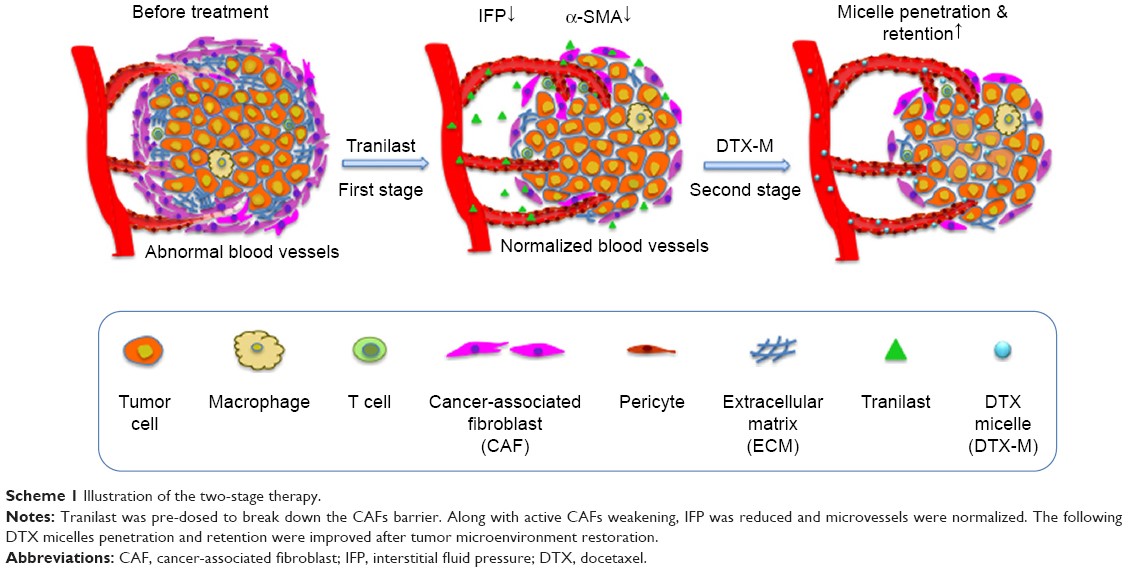
Keywords: two-stage therapy, tumor microenvironment normalization, cancer-associated fibroblasts, tranilast, stromal ablation
Pubmed link:https://www.ncbi.nlm.nih.gov/pubmed/30323586